Historically, the three main divisions of radiation therapy are external beam radiation therapy (EBRT or XRT) or teletherapy, brachytherapy or sealed source radiation therapy, and systemic radioisotope therapy or unsealed source radiotherapy. The differences relate to the position of the radiation source; external is outside the body, brachytherapy uses sealed radioactive sources placed precisely in the area under treatment, and systemic radioisotopes are given by infusion or oral ingestion. Brachytherapy can use temporary or permanent placement of radioactive sources. The temporary sources are usually placed by a technique called afterloading. In afterloading a hollow tube or applicator is placed surgically in the organ to be treated, and the sources are loaded into the applicator after the applicator is implanted. This minimizes radiation exposure to health care personnel. Particle therapy is a special case of external beam radiation therapy where the particles are protons or heavier ions. Intraoperative radiation therapy or IORT is a special type of radiation therapy that is delivered immediately after surgical removal of the cancer. This method has been employed in breast cancer (TARGeted Introperative radiation therapy or TARGIT), brain tumors and rectal cancers.
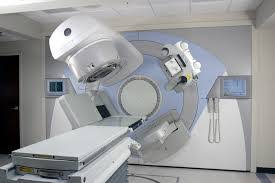
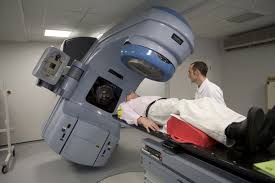
External beam radiation therapy
The following three sections refer to treatment using x-rays.
Conventional external beam radiation therapy
Conventional external beam radiation therapy (2DXRT) is delivered via two-dimensional beams using kilovoltage therapy x-ray units or medical linear accelerators which generate high energy x-rays. 2DXRT mainly consists of a single beam of radiation delivered to the patient from several directions: often front or back, and both sides. Conventional refers to the way the treatment is planned or simulated on a specially calibrated diagnostic x-ray machine known as a simulator because it recreates the linear accelerator actions (or sometimes by eye), and to the usually well-established arrangements of the radiation beams to achieve a desired plan. The aim of simulation is to accurately target or localize the volume which is to be treated. This technique is well established and is generally quick and reliable. The worry is that some high-dose treatments may be limited by the radiation toxicity capacity of healthy tissues which lie close to the target tumor volume. An example of this problem is seen in radiation of the prostate gland, where the sensitivity of the adjacent rectum limited the dose which could be safely prescribed using 2DXRT planning to such an extent that tumor control may not be easily achievable. Prior to the invention of the CT, physicians and physicists had limited knowledge about the true radiation dosage delivered to both cancerous and healthy tissue. For this reason, 3-dimensional conformal radiation therapy is becoming the standard treatment for a number of tumor sites. More recently other forms of imaging are used including MRI, PET, SPECT and Ultrasound.
Stereotactic radiation
Stereotactic radiation is a specialized type of external beam radiation therapy. It uses focused radiation beams targeting a well-defined tumor using extremely detailed imaging scans. Radiation oncologists perform stereotactic treatments, often with the help of a neurosurgeon for tumors in the brain or spine.
There are two types of stereotactic radiation. Stereotactic radiosurgery (SRS) is when doctors use a single or several stereotactic radiation treatments of the brain or spine. Stereotactic body radiation therapy (SBRT) refers to one or several stereotactic radiation treatments with the body, such as the lungs.
Some doctors say an advantage to stereotactic treatments is that they deliver the right amount of radiation to the cancer in a shorter amount of time than traditional treatments, which can often take 6 to 11 weeks. Plus treatments are given with extreme accuracy, which should limit the effect of the radiation on healthy tissues. One problem with stereotactic treatments is that they are only suitable for certain small tumors.
Stereotactic treatments can be confusing because many hospitals call the treatments by the name of the manufacturer rather than calling it SRS or SBRT. Brand names for these treatments include Axesse, Cyberknife, Gamma Knife, Novalis, Primatom, Synergy, X-Knife, TomoTherapy, Trilogy and Truebeam. This list changes as equipment manufacturers continue to develop new, specialized technologies to treat cancers.
Virtual simulation, and 3-dimensional conformal radiation therapy
The planning of radiation therapy treatment has been revolutionized by the ability to delineate tumors and adjacent normal structures in three dimensions using specialized CT and/or MRI scanners and planning software.
Virtual simulation, the most basic form of planning, allows more accurate placement of radiation beams than is possible using conventional X-rays, where soft-tissue structures are often difficult to assess and normal tissues difficult to protect.
An enhancement of virtual simulation is 3-dimensional conformal radiation therapy (3DCRT), in which the profile of each radiation beam is shaped to fit the profile of the target from a beam’s eye view (BEV) using a multileaf collimator (MLC) and a variable number of beams. When the treatment volume conforms to the shape of the tumor, the relative toxicity of radiation to the surrounding normal tissues is reduced, allowing a higher dose of radiation to be delivered to the tumor than conventional techniques would allow.
Intensity-modulated radiation therapy (IMRT)
Intensity-modulated radiation therapy (IMRT) is an advanced type of high-precision radiation that is the next generation of 3DCRT. IMRT also improves the ability to conform the treatment volume to concave tumor shapes, for example when the tumor is wrapped around a vulnerable structure such as the spinal cord or a major organ or blood vessel. Computer-controlled x-ray accelerators distribute precise radiation doses to malignant tumors or specific areas within the tumor. The pattern of radiation delivery is determined using highly tailored computing applications to perform optimization and treatment simulation (Treatment Planning). The radiation dose is consistent with the 3-D shape of the tumor by controlling, or modulating, the radiation beam’s intensity. The radiation dose intensity is elevated near the gross tumor volume while radiation among the neighboring normal tissues is decreased or avoided completely. This results in better tumor targeting, lessened side effects, and improved treatment outcomes than even 3DCRT.
3DCRT is still used extensively for many body sites but the use of IMRT is growing in more complicated body sites such as CNS, head and neck, prostate, breast, and lung. Unfortunately, IMRT is limited by its need for additional time from experienced medical personnel. This is because physicians must manually delineate the tumors one CT image at a time through the entire disease site which can take much longer than 3DCRT preparation. Then, medical physicists and dosimetrists must be engaged to create a viable treatment plan. Also, the IMRT technology has only been used commercially since the late 1990s even at the most advanced cancer centers, so radiation oncologists who did not learn it as part of their residency programs must find additional sources of education before implementing IMRT.
Proof of improved survival benefit from either of these two techniques over conventional radiation therapy (2DXRT) is growing for many tumor sites, but the ability to reduce toxicity is generally accepted. This is particularly the case for head and neck cancers in a series of pivotal trials performed by Professor Christopher Nutting of the Royal Marsden Hospital. Both techniques enable dose escalation, potentially increasing usefulness. There has been some concern, particularly with IMRT, about increased exposure of normal tissue to radiation and the consequent potential for secondary malignancy. Overconfidence in the accuracy of imaging may increase the chance of missing lesions that are invisible on the planning scans (and therefore not included in the treatment plan) or that move between or during a treatment (for example, due to respiration or inadequate patient immobilization). New techniques are being developed to better control this uncertainty—for example, real-time imaging combined with real-time adjustment of the therapeutic beams. This new technology is called image-guided radiation therapy (IGRT) or four-dimensional radiation therapy.
Another technique is the real-time tracking and localization of one or more small implantable electric devices implanted inside or close to the tumor. There are various types of medical implantable devices that are used for this purpose. It can be a magnetic transponder which senses the magnetic field generated by several transmitting coils, and then transmits the measurements back to the positioning system to determine the location. The implantable device can also be a small wireless transmitter sending out an RF signal which then will be received by a sensor array and used for localization and real-time tracking of the tumor position.
Volumetric modulated arc therapy (VMAT)
Volumetric modulated arc therapy (VMAT) is a new radiation technique, which can achieve highly conformal dose distributions on target volume coverage and sparing of normal tissues. The specificity of this technique is to modify the three parameters during the treatment. VMAT delivers radiation by rotating gantry (usually 360° rotating fields with one or more arcs), changing speed and shape of the beam with a multileaf collimator (MLC) (“sliding window” system of moving) and fluence output rate (dose rate) of the medical linear accelerator. VMAT also has the potential to give additional advantages in patient treatment, such as reduced delivery time of radiation, compared with conventional static field intensity modulated radiotherapy (IMRT).
Particle therapy
In particle therapy (proton therapy being one example), energetic ionizing particles (protons or carbon ions) are directed at the target tumor. The dose increases while the particle penetrates the tissue, up to a maximum (the Bragg peak) that occurs near the end of the particle’s range, and it then drops to (almost) zero. The advantage of this energy deposition profile is that less energy is deposited into the healthy tissue surrounding the target tissue.
Auger therapy
Auger therapy (AT) makes use of a very high dose of ionizing radiation in situ that provides molecular modifications at an atomic scale. AT differs from conventional radiation therapy in several aspects; it neither relies upon radioactive nuclei to cause cellular radiation damage at a cellular dimension, nor engages multiple external pencil-beams from different directions to zero-in to deliver a dose to the targeted area with reduced dose outside the targeted tissue/organ locations. Instead, the in situ delivery of a very high dose at the molecular level using AT aims for in situ molecular modifications involving molecular breakages and molecular re-arrangements such as a change of stacking structures as well as cellular metabolic functions related to the said molecule structures.
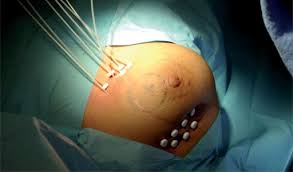
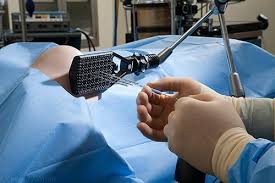
IORT
Intraoperative radiotherapy
Intraoperative radiation therapy (IORT) is applying therapeutic levels of radiation to a target area, such as a cancer tumor, while the area is exposed during surgery. The goal of IORT is to improve local tumor control and survival rates for patients with different types of cancer.
Rationale
The rationale for IORT is to deliver a high dose of radiation precisely to the targeted area with minimal exposure of surrounding tissues which are displaced or shielded during the IORT. Conventional radiation techniques such as external beam radiotherapy (EBRT) following surgical removal of the tumor have several drawbacks: The tumor bed where the highest dose should be applied is frequently missed due to the complex localization of the wound cavity even when modern radiotherapy planning is used. Additionally, the usual delay between the surgical removal of the tumor and EBRT may allow a repopulation of the tumor cells. These potentially harmful effects can be avoided by delivering the radiation more precisely to the targeted tissues leading to immediate sterilization of residual tumor cells. Another aspect is that wound fluid has a stimulating effect on tumor cells. IORT was found to inhibit the stimulating effects of wound fluid.
IORT in Breast Cancer
The largest experience with IORT and the best evidence for its potentials exists in breast cancer where a substantial number of patients have already been treated using, for example, the targeted intra-operative radiotherapy (TARGIT) technique.
On 11 November 2013 the 5-year results of local recurrence and overall survival from the TARGIT-A trial of TARGIT IORT for breast cancer were published in the Lancet. 3451 patients from 33 centres in 11 countries participated in the trial. The analysis of the data found that
- with longer follow up, the results are stable,
- local recurrence in the conserved breast with TARGIT concurrent with lumpectomy is similar to whole breast radiotherapy,
- breast cancer mortality is similar with TARGIT and EBRT, and
- deaths from causes other than breast cancer- cardiovascular and other cancers – are significantly reduced.
The conclusion was that TARGIT concurrent with lumpectomy within a risk-adapted approach should be considered as an option for eligible patients with breast cancer carefully selected as per the TARGIT-A trial protocol, as an alternative to postoperative EBRT. The results of TARGIT TARGIT IORT for breast cancer are discussed in a podcast of the TARGIT-A and ELIOT trials on the Lancet website. (full TARGIT IORT paper).
Deep inspiration breath-hold
Deep inspiration breath-hold (DIBH) is a method of delivering radiotherapy while limiting radiation exposure to the heart and lungs. It is used primarily for treating left-sided breast cancer. The technique involves a patient holding their breath during treatment. There are two basic methods of performing DIBH: free-breathing breath-hold and spirometry-monitored deep inspiration breath hold.